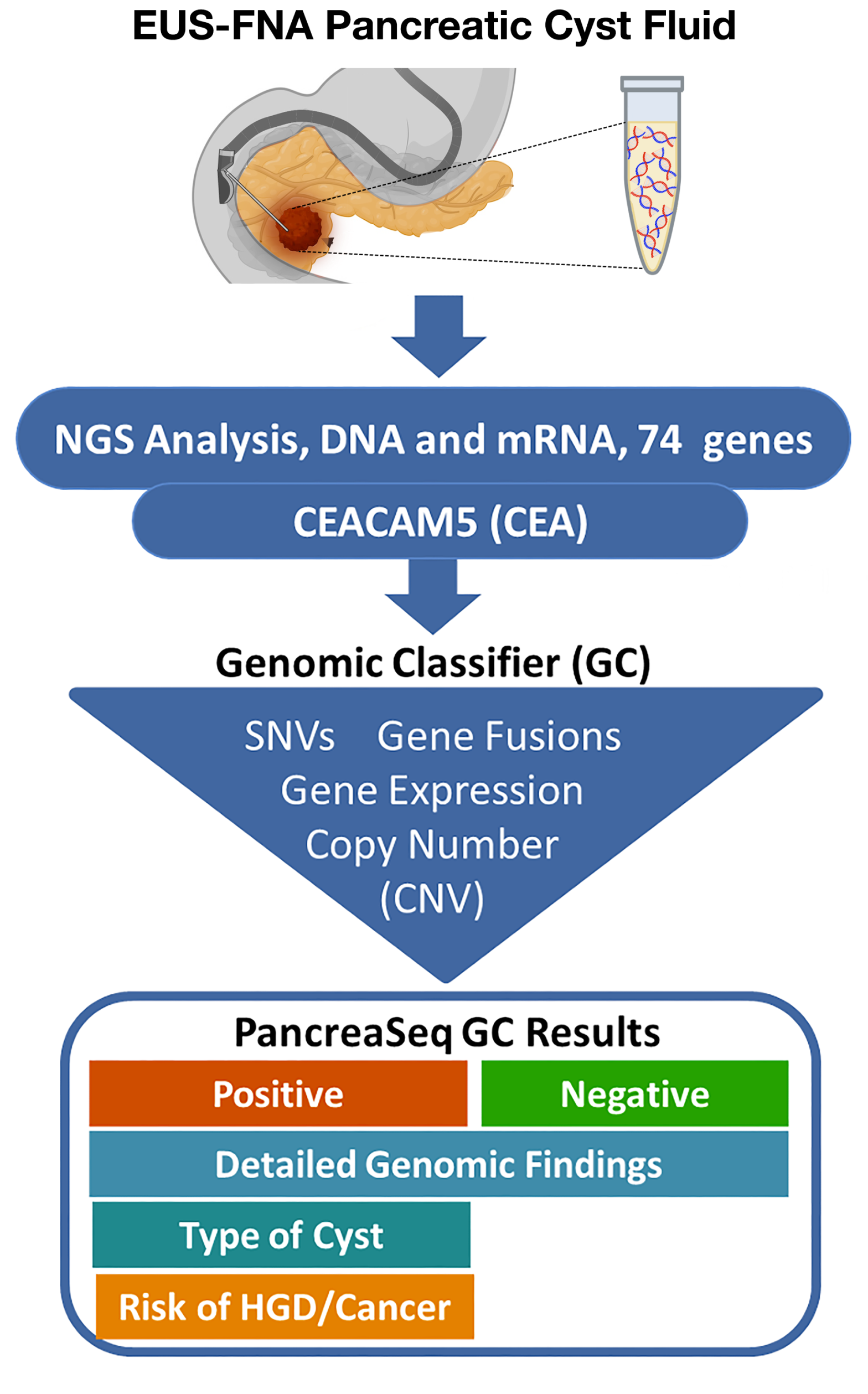
PancreaSeq® GC is performed using a small amount of pancreatic cyst fluid collected into preservative solution during ultrasound-fine needle aspiration (EUS-FNA) procedure.
It performs next-generation sequencing to analyze DNA and RNA of 74 pancreatic cyst- and pancreatic cancer-related genes for main classes of molecular alterations, including mutations, gene fusions, copy number alterations, and gene expression alterations. In addition, it detects expression of the CEA (CEACAM5) gene.
The results are processed by a unique Genomic Classifier (GC) and reported as Positive for neoplastic cyst, with prediction of cyst type and associated risk of progression to high grade dysplasia and cancer, or Negative for neoplastic cyst. In addition, it provides a detailed report on individual molecular alterations and CEA (CEACAM5) gene expression level.
KRAS, GNAS, BRAF, VHL, TP53, PIK3CA, PTEN, CTNNB1, MEN1, AKT1, APC, HRAS, NRAS, IDH1, IDH2, MET, NF2, STK11, TERT, TSC2
RNF43 (17q), SMAD4 (18q), TP53 (17p), VHL (3p), NF2 (22q), and PTEN (10q) tumor suppressor genes and 13 other chromosomal regions
Including PRKACA/B fusions for detection of IOPN, and ALK, NTRK1/3, BRAF, RET gene fusions as potential therapeutic targets for PDAC
KRT7, KRT20, CHGA, CEACAM5, PGK

PancreaSeq is a product of the Molecular & Genomic Pathology Laboratory at the University of Pittsburgh Medical Center.