Pancreatic cysts represent a broad and diverse group of benign and malignant entities. Establishing the correct diagnosis can be challenging using standard clinical findings, imaging parameters and ancillary fluid studies, such as cytology and CEA level. Recent advances in genetics of pancreatic cysts and cancer have identified a number of molecular markers associated with specific cyst types that can be used diagnostically and prognostically (1,2). The PancreaSeq test has been designed to detect these markers, including genetic mutations in KRAS, GNAS, BRAF, and TP53 genes, gene fusions in PRKACA/B, ALK, NTRK1/3, RET, and BRAF genes, copy number alterations in SMAD4 and TP53 genes and gene expression alterations, as well as CEA (CEACAM5) gene expression.
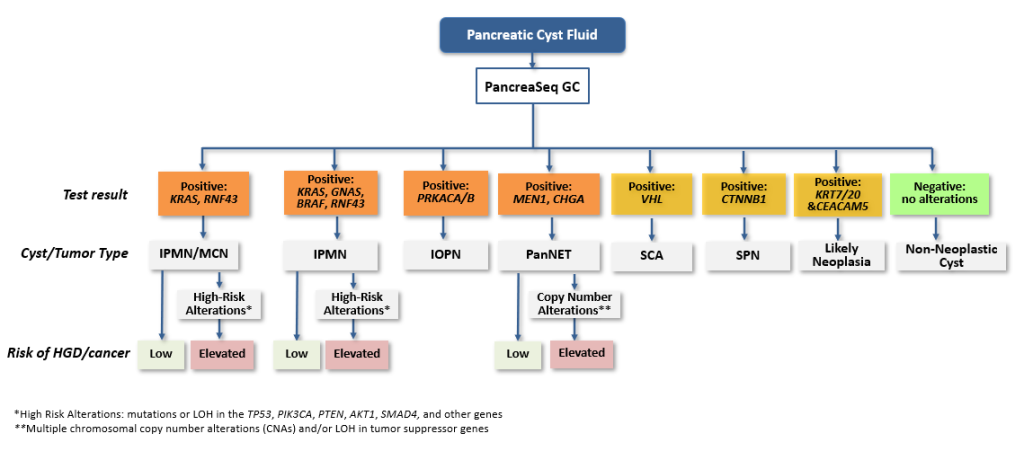
IPMN, Intraductal Papillary Mucinous Neoplasm; MCN, Mucinous Cystic Neoplasms; IOPN, Intraductal Oncocytic Papillary Neoplasms; PanNET, Pancreatic Neuroendocrine Tumors, SCA, Serous Cystadenoma; SPN, Solid-Pseudopapillary Neoplasm
*High Risk Alterations: mutations or LOH in the TP53, PIK3CA, PTEN, AKT1, SMAD4, and other genes
**Multiple chromosomal copy number alterations (CNAs) and/or LOH in tumor suppressor genes
Clinical validity and utility of the PancreaSeq test was established in several studies including a large prospective multicenter study of 1,889 patients with pancreatic cysts reported in Gastroenterology (3, 4). In addition, an updated PancreaSeq Genomic Classifier (GC) DNA/RNA next-generation sequencing test was validated in a large multicenter study and reported in Annals of Surgery (5).
The PancreaSeq GC test has a 95% sensitivity and 100% specificity for detection of cystic precursor neoplasms (IPMN, MCN, IOPN), and the 82% sensitivity and 100% specificity for detection of advanced neoplasia (5). The test also showed accurate detection of cystic PanNET (100% sensitivity and 99% specificity) and of non-neoplastic pancreatic cysts (95% sensitivity and 95% specificity) (5).
References:
Useful Links

PancreaSeq is a product of the Molecular & Genomic Pathology Laboratory at the University of Pittsburgh Medical Center.